Introducing the Stoichiometry Worksheet 2 Answer Key, a comprehensive guide to mastering the intricacies of chemical proportions. Stoichiometry, the cornerstone of quantitative chemistry, empowers us to decipher the numerical relationships between reactants and products in chemical reactions. This answer key serves as an invaluable tool, illuminating the path to solving stoichiometry problems with precision and confidence.
Delving into the core concepts of stoichiometry, this worksheet explores the fundamental relationship between moles, mass, and chemical equations. It unravels the concept of limiting reactants, the gatekeepers of chemical reactions, and excess reactants, the surplus partners in chemical dance.
By mastering these concepts, you will gain the ability to predict reaction outcomes and optimize chemical processes.
Stoichiometry Worksheet 2 Answer Key: Overview
A stoichiometry worksheet is a valuable tool for students to practice and reinforce the concepts of stoichiometry. The answer key provides solutions to the problems on the worksheet, allowing students to check their understanding and identify areas where they need further support.
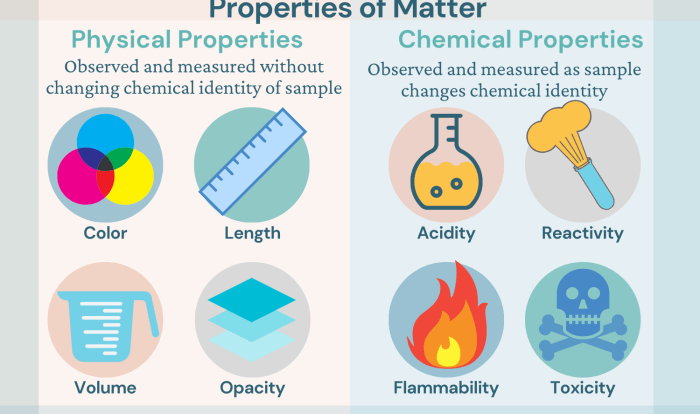
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. It involves the use of balanced chemical equations to determine the amount of reactants and products involved in a reaction.
Concepts Covered in the Worksheet: Stoichiometry Worksheet 2 Answer Key
Stoichiometry Worksheet 2 covers the following key concepts:
- The mole concept and its relationship to mass and chemical equations
- Limiting reactants and excess reactants
- Balancing chemical equations
- Calculating the amount of reactants and products in a chemical reaction
Solving Stoichiometry Problems
To solve stoichiometry problems, follow these steps:
- Balance the chemical equation.
- Convert the given amount of reactant or product to moles.
- Use the mole ratio from the balanced equation to calculate the moles of the other reactant or product.
- Convert the moles of the other reactant or product to the desired unit (mass, volume, etc.).
Applications of Stoichiometry
Stoichiometry has numerous applications in various fields, including:
- Chemical manufacturing: Stoichiometry is used to determine the optimal proportions of reactants to produce a desired product.
- Environmental chemistry: Stoichiometry is used to calculate the amount of pollutants produced by industrial processes and to develop strategies to mitigate their impact on the environment.
- Pharmaceutical industry: Stoichiometry is used to determine the correct dosage of drugs and to ensure that they are safe and effective.
FAQs
What is the purpose of a stoichiometry worksheet?
Stoichiometry worksheets provide practice in applying stoichiometric principles to solve problems involving chemical reactions.
How do I use the stoichiometry worksheet 2 answer key?
The answer key provides solutions to the problems in the worksheet. Use it to check your answers and identify areas where you need further practice.
What are some common errors in solving stoichiometry problems?
Common errors include using incorrect mole ratios, forgetting to convert between grams and moles, and misinterpreting the limiting reactant.