Welcome to the realm of chemistry, the central science that unravels the mysteries of matter and energy. In this exploration, we delve into the highly acclaimed textbook, “Chemistry: The Central Science 15th Edition PDF,” a comprehensive guide that illuminates the fundamental principles and applications of this captivating field.
The 15th edition of this renowned textbook boasts significant advancements, offering a wealth of updated content, interactive simulations, and engaging multimedia resources. Embark on a journey through the key concepts, groundbreaking discoveries, and practical applications that define the ever-evolving landscape of chemistry.
Introduction
Chemistry is the study of matter and its properties, as well as the changes it undergoes during chemical reactions. It is a central science, as it provides the foundation for understanding many other scientific disciplines, such as biology, physics, and geology.
“Chemistry: The Central Science” is a textbook that has been used by students and educators for over 50 years. The 15th edition of this textbook has been extensively revised and updated to reflect the latest advancements in the field of chemistry.
Key Features of the 15th Edition
- New and updated content on topics such as sustainability, green chemistry, and nanotechnology.
- A new chapter on the chemistry of life.
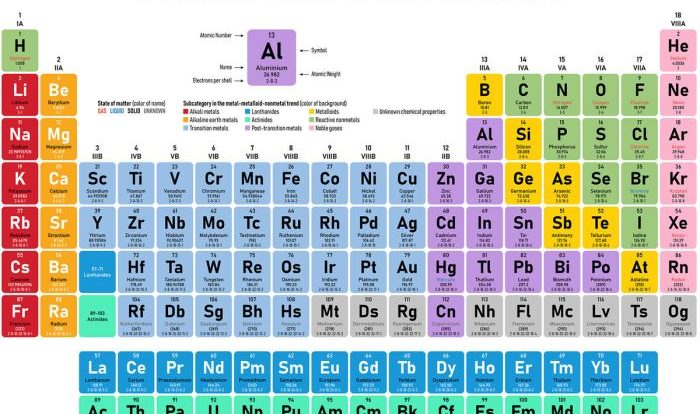
- A revised and expanded discussion of the periodic table.
- A new emphasis on problem-solving and critical thinking skills.
Key Concepts

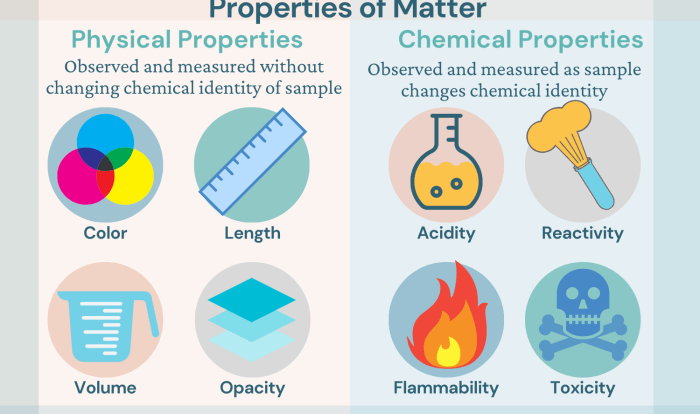
Matter and Its Properties
Matter is anything that has mass and takes up space. It can exist in three states: solid, liquid, and gas. The properties of matter are determined by the arrangement and interactions of its constituent particles.
Energy and Chemical Reactions
Energy is the ability to do work. Chemical reactions involve the rearrangement of atoms and molecules, and they can release or absorb energy.
Chemical Bonding
Chemical bonding is the force that holds atoms and molecules together. There are different types of chemical bonds, and the type of bond that forms depends on the atoms involved.
Chemical Reactions and Stoichiometry
Types of Chemical Reactions
There are many different types of chemical reactions, including:
- Combination reactions
- Decomposition reactions
- Single-replacement reactions
- Double-replacement reactions
Stoichiometry
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions.
States of Matter
Solids
Solids have a definite shape and volume. The particles in a solid are tightly packed together.
Liquids
Liquids have a definite volume but no definite shape. The particles in a liquid are loosely packed together.
Gases
Gases have no definite shape or volume. The particles in a gas are very far apart.
Phase Transitions
Phase transitions are changes in the state of matter. Phase transitions can be caused by changes in temperature, pressure, or both.
Solutions: Chemistry: The Central Science 15th Edition Pdf

Definition of a Solution
A solution is a homogeneous mixture of two or more substances. The substance that is present in the largest amount is called the solvent. The substance that is present in the smallest amount is called the solute.
Types of Solutions
There are many different types of solutions, including:
- Aqueous solutions
- Non-aqueous solutions
- Electrolyte solutions
- Non-electrolyte solutions
Solubility
Solubility is the ability of a solute to dissolve in a solvent. The solubility of a solute depends on the temperature, pressure, and nature of the solute and solvent.
Thermodynamics
Laws of Thermodynamics
The laws of thermodynamics are the fundamental laws that govern the behavior of energy in chemical reactions.
- The first law of thermodynamics states that energy cannot be created or destroyed.
- The second law of thermodynamics states that the entropy of a system always increases over time.
- The third law of thermodynamics states that the entropy of a perfect crystal at absolute zero is zero.
Entropy, Enthalpy, and Free Energy
Entropy, enthalpy, and free energy are three important thermodynamic concepts.
- Entropy is a measure of the disorder of a system.
- Enthalpy is a measure of the heat content of a system.
- Free energy is a measure of the spontaneity of a reaction.
Chemical Bonding
Types of Chemical Bonds, Chemistry: the central science 15th edition pdf
There are three main types of chemical bonds:
- Ionic bonds
- Covalent bonds
- Metallic bonds
Properties of Chemical Bonds
The properties of a chemical bond depend on the type of bond.
- Ionic bonds are strong and brittle.
- Covalent bonds are weaker and more flexible.
- Metallic bonds are strong and ductile.
Organic Chemistry
Overview of Organic Chemistry
Organic chemistry is the study of compounds that contain carbon. Organic compounds are found in all living things, and they play a vital role in many industrial processes.
Structure and Properties of Organic Molecules
The structure of an organic molecule determines its properties. Organic molecules can be classified into different functional groups, and each functional group has its own characteristic properties.
Reactions of Organic Molecules
Organic molecules can undergo a variety of reactions. The type of reaction that occurs depends on the structure of the molecule and the reaction conditions.
Applications of Chemistry
Chemistry in Medicine
Chemistry plays a vital role in the development of new drugs and treatments for diseases.
Chemistry in Industry
Chemistry is used in the production of a wide variety of products, including plastics, fertilizers, and fuels.
Chemistry in Environmental Science
Chemistry is used to study and solve environmental problems, such as pollution and climate change.
Clarifying Questions
What is the significance of “Chemistry: The Central Science” textbook?
As a comprehensive guide to chemistry, this textbook provides a solid foundation in the fundamental principles and applications of the field. Its clear explanations, engaging examples, and up-to-date content make it an invaluable resource for students and educators alike.
What are the key features of the 15th edition?
The 15th edition offers a range of advancements, including updated content, interactive simulations, and multimedia resources. It incorporates the latest discoveries and research in the field, ensuring that readers have access to the most current information.
How can I access the “Chemistry: The Central Science 15th Edition PDF”?
The accessibility of the PDF version of the textbook may vary depending on the publisher and licensing agreements. It is recommended to check with your educational institution or explore reputable online sources for authorized access.